Losh, S., Rague, R., in review, Mineral. Deposita
For over a century, the conventional wisdom about the oxidation of iron ores on Minnesota’s Mesabi Iron Range has been the “top down” model, whereby cool, fresh meteoric waters flowed downward from the surface and oxidized and leached minerals from the iron-rich sedimentary rocks (iron formation) resulting in iron ores of hematite and/or goethite that in many cases were of sufficiently high grade to ship directly to steel mills. These so-called ‘natural ores’ typically decreased in grade and lateral extent with depth below the surface, and were largely mined out by the 1970’s. They have since been supplanted in mining by the lower-grade largely unoxidized magnetite-bearing iron formation that is currently mined at several places on the Mesabi Range. In many of these mines, oxidation is localized along high-angle faults. It turns out, however, that much of this fault-related oxidation is not “top-down” (e.g., supergene, near the surface) but rather took place at depth as a result of interaction with hydrothermal fluids.
The idea of hydrothermal oxidation of iron ores in the Mesabi Range can be traced to Gruner’s 1956 report, in which he advanced the possibility but like nearly all others before and since attributed much of the oxidation to supergene processes. Morey (1999) proposed a model of “bottom-up” fluid flow for formation of the natural ores, whereby hot, saline basinal fluids, possibly driven upward during Paleoproterozoic (Penokean, 1.83 – 1.87 Ga) collisional tectonics, flowed upward along high-angle faults. However, he provided no solid evidence for hydrothermal oxidation in these rocks. Hydrothermal oxidation of iron ores, commonly accompanied by dissolution of non-iron oxide minerals (namely quartz, carbonates, and iron silicates) is known from other parts of the world (e.g., Australia, Brazil), so the idea is worthy of consideration for the ores in Minnesota. If true, the “bottom up” flow model could point the way to deeper, high-grade direct-shipping ore. In addition, better understanding of the ‘plumbing system’ related to these iron ores could lead to ability to predict the occurrence of fine-grained silica that is common in magnetite from oxidized zones, a problem that plagues many of the producers on the Range. My students and I are the first to demonstrate that hydrothermal (relatively high temperature) oxidation has occurred in these rocks: it is involved in silica redistribution, but it is not likely responsible for the formation of natural ores.
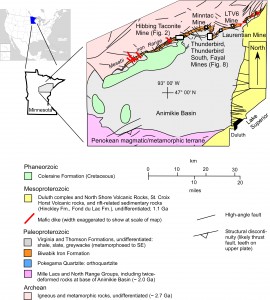
To study the fluid flow systematics related to oxidation of iron formation, we utilized petrographic, fluid inclusion, cathodoluminescence, SEM, and bulk geochemical techniques. We sampled traverses from quartz fault breccias through adjacent oxidized iron formation into unoxidized iron formation in several places in the Hibbing Taconite Mine, as well as from the Thunderbird, Fayal, Laurentian, Minntac, and LTV6 mines (Figure 1). To place the faults and the fluids they hosted into a broader paragenetic context, we sampled various low-angle and high-angle vein sets, with and without associated oxidation and with various mineral assemblages.
Figure 1. Geologic map of Mesabi Iron Range, northern Minnesota, showing locations of sampled mines. Map from Jirsa et al. (2011, Minn. Geol Surv Map Series)
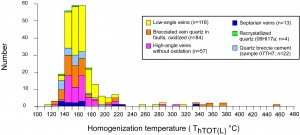
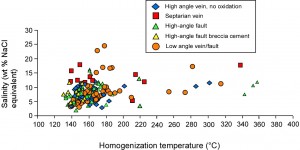
Fluid inclusion data show that the fluids in all the veins, including quartz cement in fault breccia, had elevated temperatures, averaging 155° C (homogenization temperature, which is less than the actual fluid temperature at the time of mineral growth; Figure 2). These temperatures are consistent with oxygen isotope estimates of 150°-200° C for diagenesis of iron formation (Perry et al., 1973), which produced the assemblage quartz, minnesotaite (Fe talc), greenalite (Fe serpentine), chamosite (Fe chlorite), carbonates (calcite, ankerite, and siderite), magnetite, pyrite, and minor hematite. Diagenetic fluids, sampled in septaria from the Thunderbird Mine, had salinities (saltiness) of 12 – 19 weight percent NaCl equivalent (see Figure 3). Fluids in the various kinds of veins as well as in the high-angle faults associated with oxidation, had salinites that ranged from this high diagenetic value to considerably lower values of about 3%, suggesting mixing of deep diagenetic fluids that were present in the sediment as it was buried with shallow-sourced meteoric fluids that may have accessed the then-buried iron formation via high-angle faults. Next to the faults, these mixed fluids then interacted with adjacent iron formation.
Figure 2. Homogenization temperatures of fluid inclusions from studied mines.
Figure 3. Salinity vs homogenization temperatures, fluid inclusions from studied mines.
What did these fluids do to the iron formation? In contrast to the supergene (“top down”) fluids that dissolved the fine-grained quartz (chert) and pretty much everything else that wasn’t iron oxide, these hot fluids did not dissolve quartz but rather recrystallized it at the same time it oxidized iron silicates to mats of acicular goethite crystals (FIgures 4a, 4b). The intimate intergrowths of goethite and recrystallized quartz is the “smoking gun” that points to a very different style of fluid-rock interaction than that exhibited by the “take no prisoners” dissolution effect of the supergene fluids. Supergene weathering does not produce these sorts of textures; hydrothermal fluid-rock interaction does.
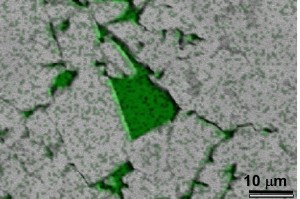
(Figure 4a, Left) Fine-grained cherty peloids in unoxidized iron formation. (Figure 4b, Right) Recrystallized quartz (Qz) intergrown with Goethite (Gth) in oxidized iron formation.
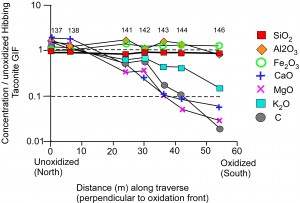
Rather than remove silica as is typically the case in “top-down” (supergene) settings, the hydrothermal oxidation documented here remobilized it. This is shown not only by quartz recrystallization (above) but by geochemical conservation of silica with increasing oxidation adjacent to faults in the Hibbing Taconite Mine (Figure 5). Other soluble components (CaO, MgO, K2O) show substantial loss with increasing oxidation in traverses from unoxidized to oxidized taconite, but SiO2 remains constant and behaves much like immobile Al2O3 in the traverses.
Figure 5. Whole-rock geochemical traverse through blast-hole pattern from unoxidized rocks (gray in powdered form, on left) to oxidized rocks (red-orange in powdered form, on right).
Time and again, “cherty” iron formation in which iron silicates, which are present in iron formation beyond the oxidation front, have been oxidized to goethite also show recrystallization of chert-textured peloids to coarser intergrown crystalline quartz grains. In some instances, those recrystallized quartz grains form haloes around magnetite and give way to mats of intergrown acicular goethite + quartz. This is not a diagenetic texture nor a psuedomorphic one (e.g., goethite directly replacing iron silicate such as minnesotaite) but rather one that formed as a result of wholesale recrystallization: minnesotaite oxidation produces nearly equal volumes of goethite and quartz, which are intimately intergrown.
Silica was remobilized during hydrothermal oxidation, leading to its being precipitated into microfractures and pit-like inclusions in magnetite (Figure 6,7)
Figure 6. Reflected light photomicrograph of fractured magnetite (fractures shown by arrows), Thunderbird Mine, near low-angle fault in LUC1 unit. Microfractures and ‘micropits’ exclusive of diagenetic quartz inclusions (e.g., straight-sided) constitute 11% of the magnetite by area.
Figure 7. SEM X ray spectra image of magnetite (gray) and silica (green), showing inclusions and microfractures that contain quartz. These features are much smaller than the 44 micron size to which the ore is ground prior to magnetic separation, and result in excess silica being incorporated into taconite pellets made from the ore.
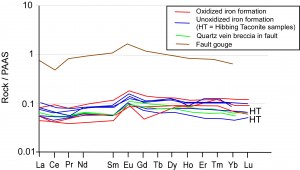
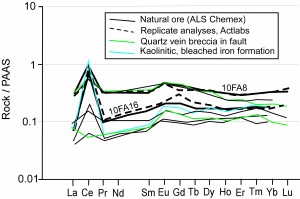
Geochemical data show that the faults along which oxidizing fluids flowed did not tap large volumes of externally-derived fluids (e.g., from basin-scale orogenesis) but rather locally-buffered fluids. Rare earth elements in particular show the same distinctive Eu-anomalous patterns in fault rocks as in oxidized and unoxidized iron formation (Figure 8). Furthermore, iron formation and fault breccia from the Fayal Reserve Mine (Figure 9) show distinctive positive Ce anomalies indicative of strongly-oxidizing, near surface conditions during ore weathering. Again, the fault tapped the same fluids that were present during oxidation of the ore. These near-surface fluids were not present in the hydrothermally-oxidized iron formation from the other mines.
Figure 8. REE for iron formation and fault samples from Thunderbird and Hibbing Taconite Mines. All show the distinctive positive Eu anomaly characteristic of many iron formations.
Figure 9. REE from the Fayal Reserve Mine. This mine produced ‘natural ore’ – high-grade hematite/goethite that resulted from near-surface (supergene) alteration of iron formation. The REE pattern is distinct from the oxidized samples in Fig. 8.